It is thought that for the time from 3.5 - 1 billion years ago that stromatolites dominated the shallow seas around continents.
Stromatolites are made up of photosynthetic bacteria that form a colony by sticking together with mucous. Sediments from the water also stick to the mucous. As the cells divide the new cells that form on top block the light out from those below. While these die, they contribute to the upward growth of the stromatolite forming a column.
Stromatolites are by far the oldest non-microscopic fossil found on Earth. The oldest are found in the Pilburra region of Western Australia and are thought to be around 3.4 billion years old.
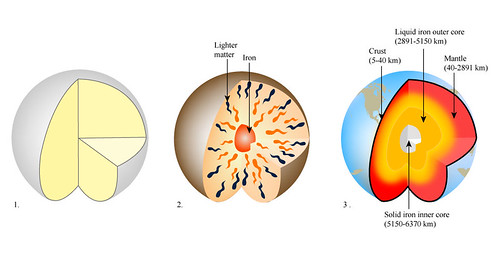
The early Earth's oceans had a lot of dissolved iron in them. From space, the oceans would have appeared green from all the iron, rather than the blue of many famous space images. Iron can stay dissolved in water if no oxygen is present. When dissolved iron comes into contact with water, it chemically combines to form solid iron oxide. When that happens, the iron settles out of solution.

This iron was thought to enter water from volcanic vents where water mixed with rising magma. The water would dissolve some of the iron from the magma and would heated up. As a result, jets of iron rich super-heated water would enter the oceans. Some of these exist at mid-ocean ridges today. The black plume is from iron compounds in the water.
So as the iron rich water circulated and came to the areas where stromatolites (and therefore oxygen) were present, it would react, form a solid and settle out forming an iron rich sediment layer (see image above). When either the iron or the oxygen was exhausted from an area, it would mean that a layer of iron poor sediment would be placed on that.

The reasons for this layering of iron rich and iron poor sediments has been subject to a number of hypotheses. These include:
1. Cycling of ocean currents replenishing the water with iron after its removal from the water by oxygen.
2. Seasonal variations of temperature which affects the rate of photosynthesis and therefore oxygen levels
3. Death and regrowth of stromatolite bacteria from oxygen poisoning. The bacteria die as oxygen rises. Any remaining oxygen is removed by the iron. The bacteria regrow and oxygen levels rise. The cycle starts again.
About 1.5 billion years ago (other estimates are earlier and vary), the formation of banded iron stopped. This is because the iron levels in the oceans were near exhaustion and the new iron entering the oceans was reaction in oxygen rich waters which were deeper and well away from stromatolites. As a result oxygen could now accumulate in the atmosphere and this again would lead to dramatic changes in the chemical makeup of the planet.
Stromatolites are made up of photosynthetic bacteria that form a colony by sticking together with mucous. Sediments from the water also stick to the mucous. As the cells divide the new cells that form on top block the light out from those below. While these die, they contribute to the upward growth of the stromatolite forming a column.
Stromatolites are by far the oldest non-microscopic fossil found on Earth. The oldest are found in the Pilburra region of Western Australia and are thought to be around 3.4 billion years old.
The early Earth's oceans had a lot of dissolved iron in them. From space, the oceans would have appeared green from all the iron, rather than the blue of many famous space images. Iron can stay dissolved in water if no oxygen is present. When dissolved iron comes into contact with water, it chemically combines to form solid iron oxide. When that happens, the iron settles out of solution.

This iron was thought to enter water from volcanic vents where water mixed with rising magma. The water would dissolve some of the iron from the magma and would heated up. As a result, jets of iron rich super-heated water would enter the oceans. Some of these exist at mid-ocean ridges today. The black plume is from iron compounds in the water.
So as the iron rich water circulated and came to the areas where stromatolites (and therefore oxygen) were present, it would react, form a solid and settle out forming an iron rich sediment layer (see image above). When either the iron or the oxygen was exhausted from an area, it would mean that a layer of iron poor sediment would be placed on that.

The reasons for this layering of iron rich and iron poor sediments has been subject to a number of hypotheses. These include:
1. Cycling of ocean currents replenishing the water with iron after its removal from the water by oxygen.
2. Seasonal variations of temperature which affects the rate of photosynthesis and therefore oxygen levels
3. Death and regrowth of stromatolite bacteria from oxygen poisoning. The bacteria die as oxygen rises. Any remaining oxygen is removed by the iron. The bacteria regrow and oxygen levels rise. The cycle starts again.
About 1.5 billion years ago (other estimates are earlier and vary), the formation of banded iron stopped. This is because the iron levels in the oceans were near exhaustion and the new iron entering the oceans was reaction in oxygen rich waters which were deeper and well away from stromatolites. As a result oxygen could now accumulate in the atmosphere and this again would lead to dramatic changes in the chemical makeup of the planet.